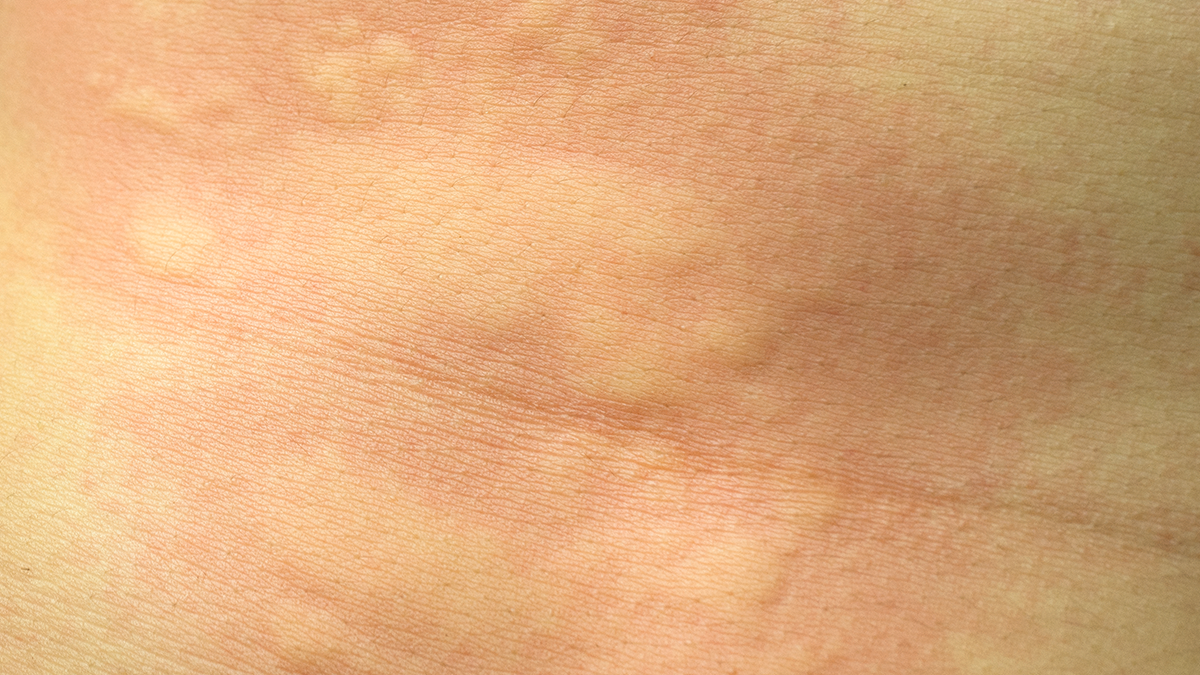
The prevalence of Allergic Rhinitis in OSA/SDB is considerably high and children with SDB suffering from a higher incidence of AR than non-SDB. OSA adults accompanied with AR do not have any influences on sleep parameters.
Allergic rhinitis (AR); obstructive sleep apnea (OSA); sleep-disordered breathing (SDB).