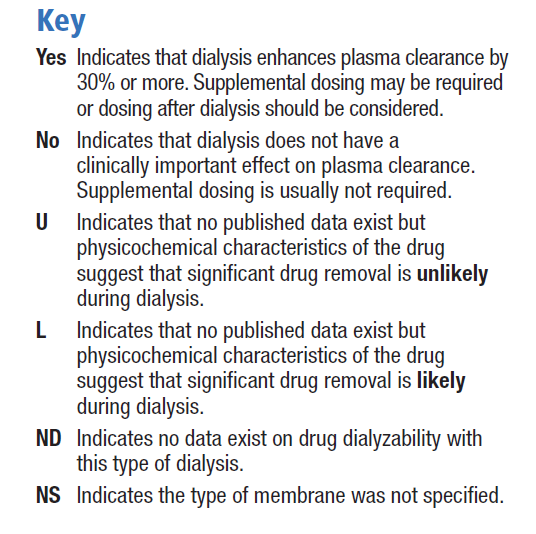
The extent to which a drug is affected by dialysis is determined primarily by its physicochemical characteristics such as molecular size and water solubility, and its pharmacokinetic properties such as absorption, plasma clearance, protein binding and volume of distribution. In addition to these properties of the drug, technical aspects of the dialysis procedure may also determine the extent to which a drug is removed by dialysis [30]. Unfortunately, few in vivo studies have been published, and the pharmacokinetic behaviour of only a small number of drugs has been investigated in dialysis patients. Therefore, many guidelines for drug dosing during continuous renal replacement therapy (CRRT) are extrapolated from experiences with chronic haemodialysis or from theoretical considerations [30]. The Dialysis of Drugs guidelines includes an accompanying table which is a reference regarding the effect of dialysis on drug clearance. The table includes antihistamines such as cetirizine, desloratadine and fexofenadine, but as bilastine is a new drug, there are no specific studies or recommendations. We consider its physicochemical characteristics to be similar to those of fexofenadine, and therefore the dialysability of bilastine might be similar to that of fexofenadine, although there are no data on dialysed patients being administered bilastine. The Dialysis of Drugs guidelines 2013 recommendations [30] for fexofenadine are conventional haemodialysis: NO/NS, NO indicates that dialysis does not have a clinically important effect on plasma clearance and supplemental dosing is not usually required / NS indicates that the type of membrane was not specified; high permeability haemodialysis: ND indicates that no data exist on drug dialysability with type of dialysis; peritoneal dialysis: U indicates significant drug removal is unlikely based on physicochemical characteristics of the drug such as protein binding, molecular size, or volume of distribution.

![]()




