Esther Serra, Cristina Campo, Zoltan Novak, Bernadetta Majorek-Olechowska, Grazyna Pulka, Aintzane García-Bea and Luis Labeaga
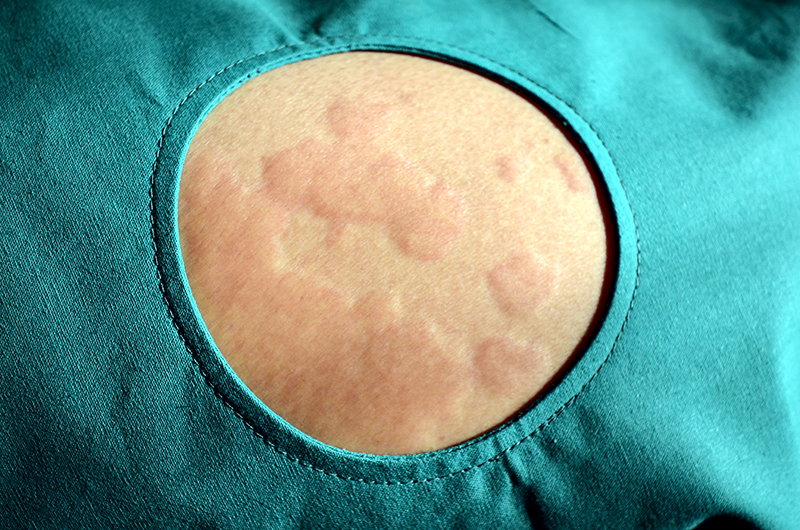
Pruritus is a common symptom associated with different skin diseases, including urticaria, atopic dermatitis, eczema and prurigo. Pruritus may have a significant impact on the quality of life and psychosocial wellbeing of patients with skin urticaria. Bilastine is a H1-antihistamine with demonstrable efficacy for the symptomatic treatment of chronic spontaneous urticaria.
A phase IV, multicentre, open-label, exploratory study to evaluate the efficacy and safety of bilastine in reducing pruritus in patients with chronic spontaneous urticaria and other skin diseases was conducted at 10 European Centres.
115 patients between 18 and 74 years diagnosed with chronic spontaneous urticaria, eczema/dermatitis, prurigo or cutaneous pruritus who had not responded to placebo during a run-in period of 7-14 days and with, at least 4 points for the sum of itch score during the last 3 days of the run-in period were included. Patients received bilastine 20 mg once daily for 8 weeks and non-responders (<30% improvement in pruritus score at week 2), received 40 mg daily from week 2.
Bilastine reduced the mean change in weekly pruritus severity score from baseline to week 8 (primary endpoint) (overall and by disease group). Up dosed non-responders (n = 31) improved weekly pruritus severity scores from baseline to week 8. Bilastine improved the Dermatology Life Quality Index at weeks 4 and 8 (p < 0,001) in all disease groups, and the 7-day Urticaria Activity Score in CSU patients (p <0 ,001).
In conclusion, bilastine has demonstrated efficacy for the relief of pruritus associated with urticaria and other skin diseases in adults, with a very good safety profile. Also, bilastine up dosing to 40mg (double dose), for patients who did not achieve a significant improvement after 2 weeks of treatment, was efficacious without any safety concerns.