Allergic rhinitis (AR) and urticaria, also known as hives, are very frequent conditions1, although their impact is often minimised or overlooked.2
- Both AR and urticaria can affect the quality of life of children and adolescents, causing limitations on their daily activity, as well as emotional, practical and sleep disruptions. As a result, these conditions may have a negative impact on school attendance and academic performance.1
According to the European Academy of Allergy and Clinical Immunology (EAACI), rhinitis is characterised by at least two of the following nasal symptoms: rhinorrhoea, nasal congestion, repeated sneezing or itching. Depending on its pathophysiology, it is classified as allergic rhinitis, infectious rhinitis or non-allergic and non-infectious rhinitis.2
Furthermore, rhinitis is often associated with eye symptoms of allergic conjunctivitis (red eyes, tearing or itchy eyes, also called ocular pruritus), leading to what is known as rhinoconjunctivitis.2
Rhinoconjunctivitis is common in school-aged children and adolescents with an overall average prevalence of 8.5% and 14.6% in the 6-7-year age group and 13-14-year age group, respectively. The prevalence of this condition appears to be increasing, particularly among adolescents.3
The International Study of Asthma and Allergies in Childhood (ISAAC) found that in a group of patients aged 6-7 years, girls showed a lower incidence of rhinoconjunctivitis than boys. On the contrary, in a group of adolescents (13-14 years), females displayed a higher prevalence as compared to their male counterparts. None of the results in both cases showed any variation according to the region where the patients lived.4
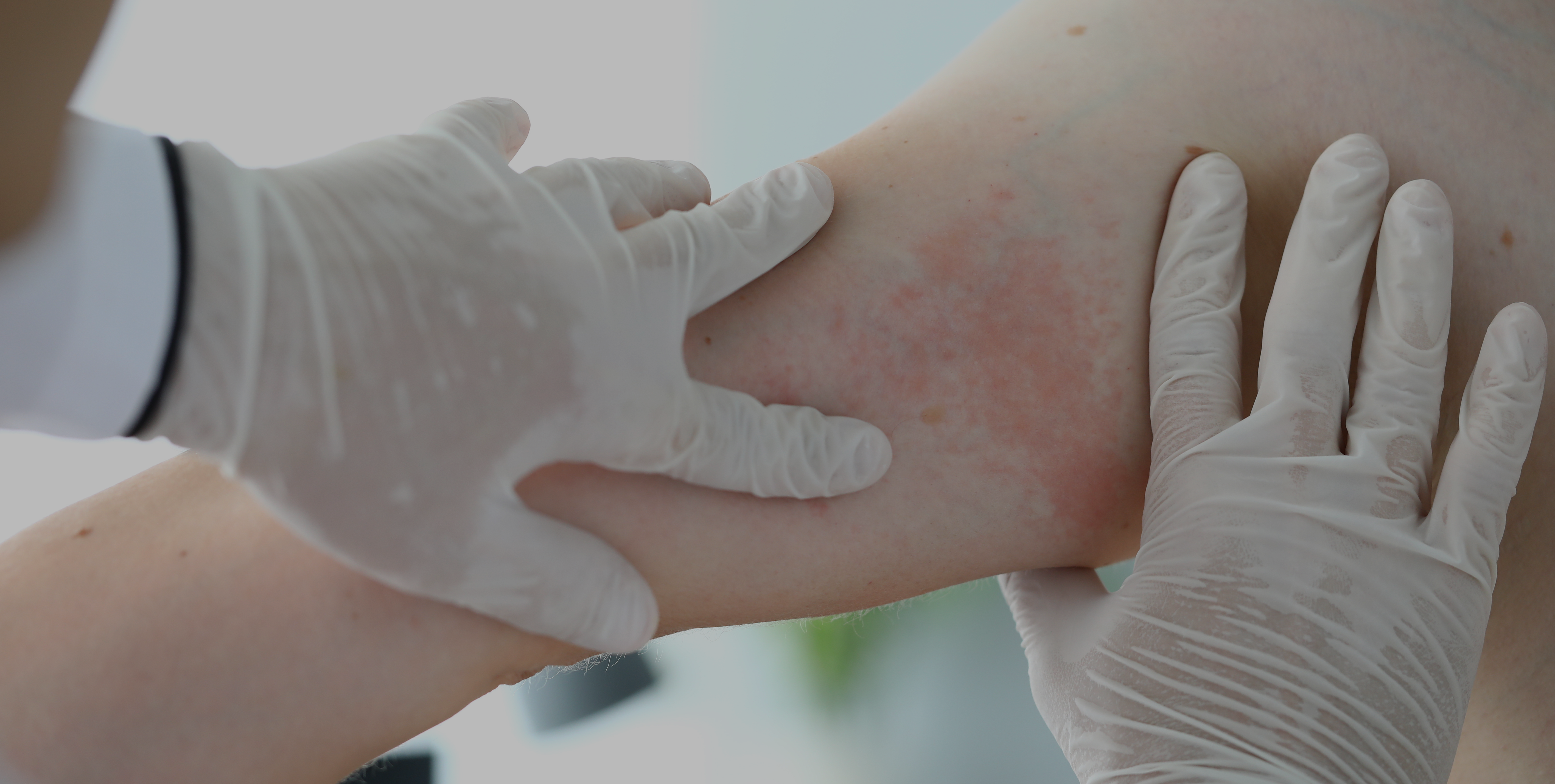
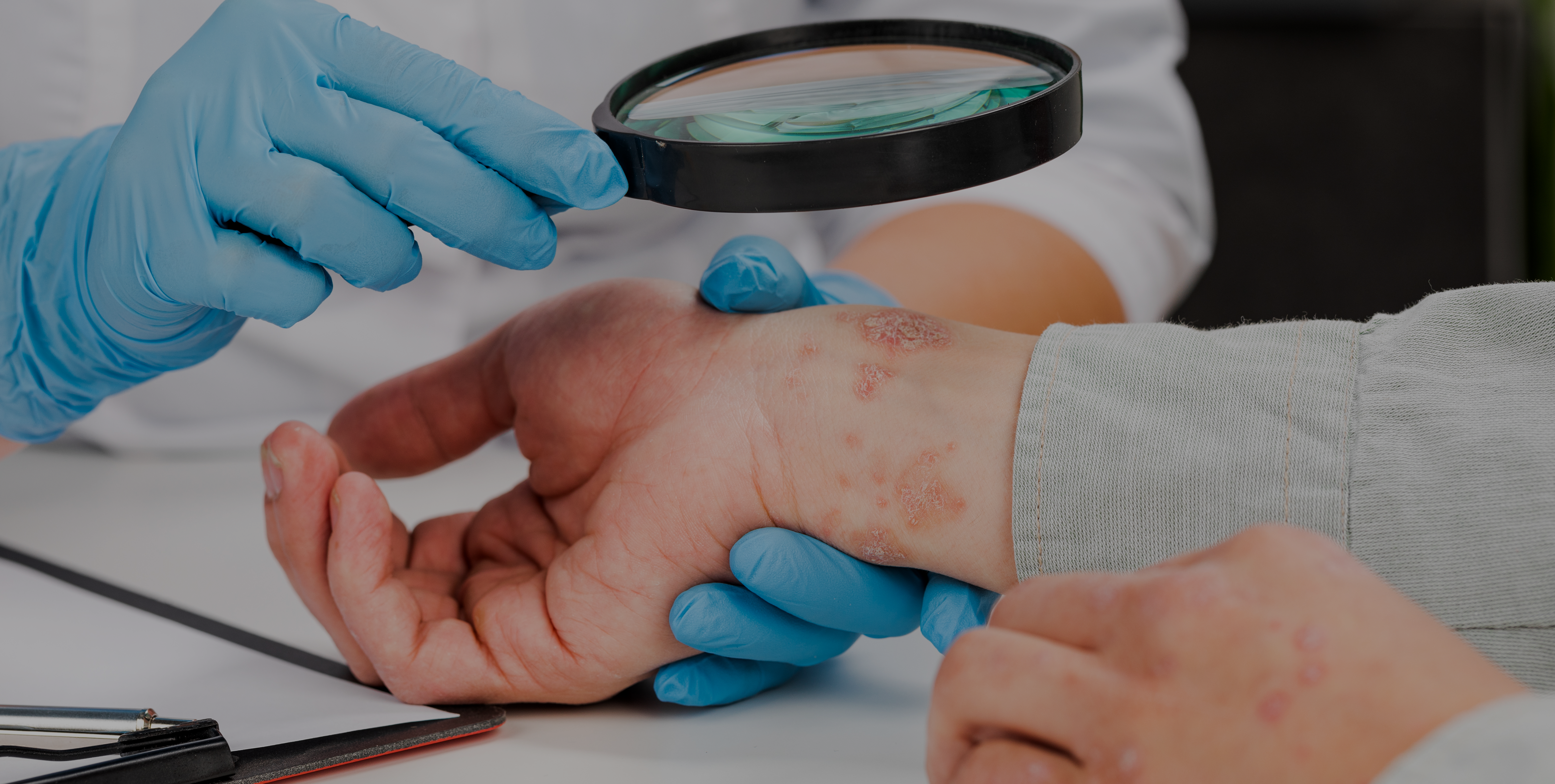
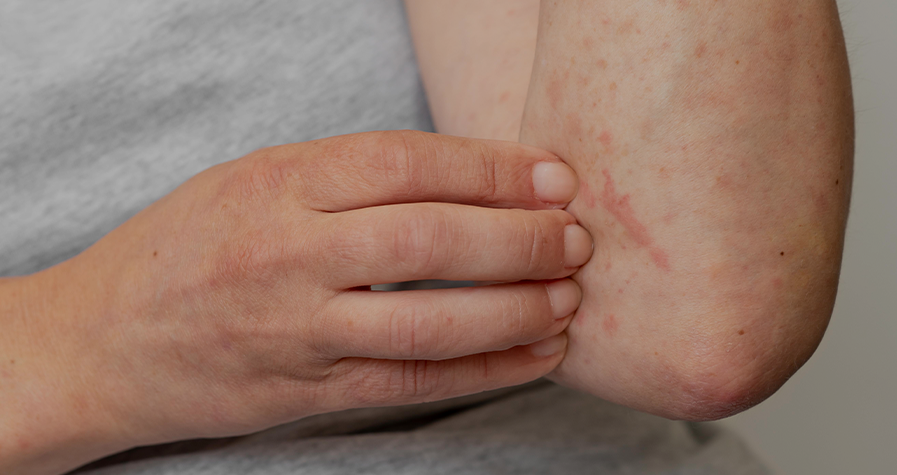
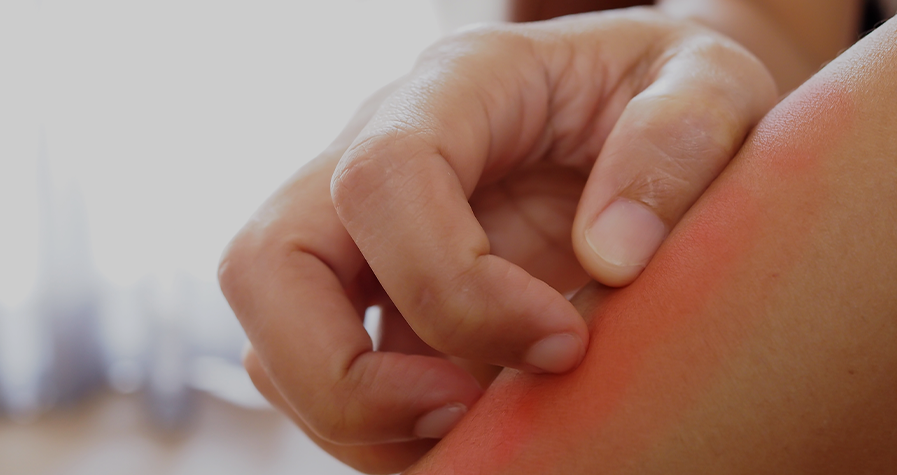
On the other hand, urticaria is characterised by the appearance of very pruritic (itchy) rashes or hives, which has a major impact on the quality of life of patients who suffer it.3 It is a highly prevalent condition, and an estimated 15-24% of the general population suffers it at some point in their lives. In paediatric patients, the prevalence of urticaria in children between 3 and 6 years is up to 43.9%.5
In children, unlike adults, acute urticaria (it remits within 6 weeks) is more common (prevalence between 1%-14% in children) than the chronic or persistent form (prevalence between 0.1%-1.8% in children).1
Acute urticaria is appears suddenly and can persist from a few hours to a maximum of 6 weeks.5 Allergic reactions to food, medicines or insect bites, viral infections, as well as anything that can trigger an immediate skin reaction are the most common causes of acute urticaria.5,6
Chronic urticaria can be caused by cold, heat, water, rubbing, among other triggering factors, but it can also arise spontaneously and there is no known cause. It is estimated that approximately half of chronic urticaria cases last less than one year, although in 11-15% of cases persistence goes beyond 5 years.5
What is the impact of these pathologies on school performance?
The symptoms of rhinitis, including sneezing, itching, nasal congestion and rhinorrhoea, disrupt sleep quality and sleep quantity, causing the child to feel sleepy during the day.7,8
- Daytime sleepiness may contribute to impair a child’s ability to concentrate, be more distracted or less attentive, affecting school performance. 8
- Moreover, sleep deprivation may lead to restlessness, irritability and moodiness in children.8
In the case of urticaria, pruritus or itching can also cause irritability and behavioural problems in children, as well as poor sleep quality and daytime drowsiness, which affect their performance at school.7 In fact, there are scales to assess the degree of disease activity according to the severity measurements, which can become intense and bothersome enough to interfere with daily activities or sleep.5