Mojgan Najafzadeh, Fanila Shahzad, Nader Ghaderi, Kaveh Ansari, Badie Jacob, Andrew Wright
J Eur Acad Dermatol Venereol . 2020 Jun 11;10.1111/jdv.16721. doi:10.1111/jdv.16721. Online ahead of print.
The relationship between urticaria and COVID-19 infection has rarely been reported, however, it has been reported that in addition to the conventional respiratory symptoms, some COVID-19 patients also have skin manifestations, such as urticaria and angioedema.
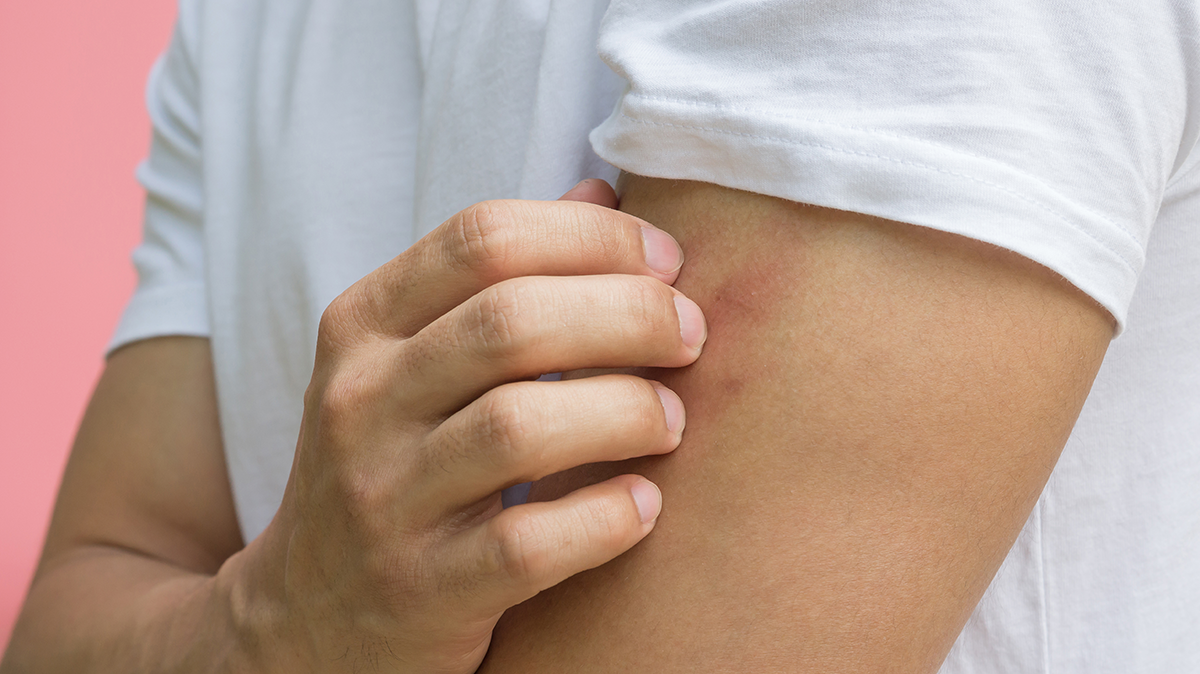
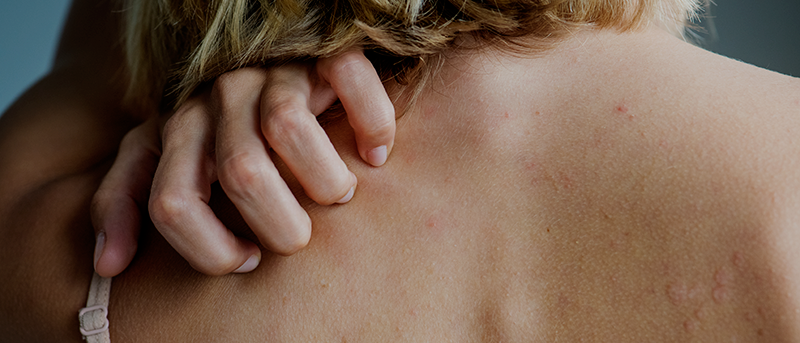
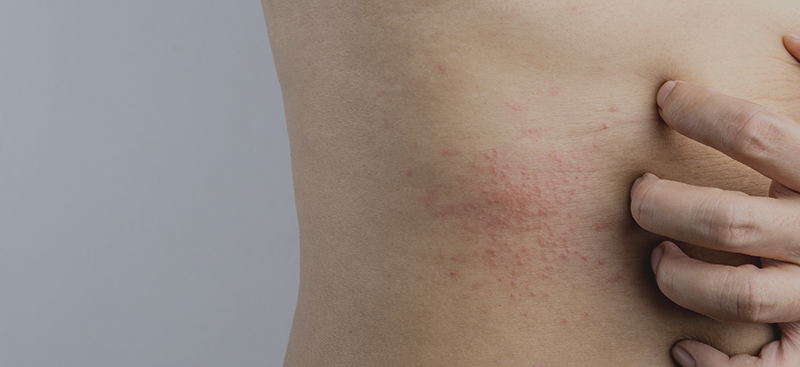
This is a case study of an elderly man who first presented with generalised pruritic hives with a diameter of 1,5 to 8,0 cm, general malaise, fatigue, temperature and sore throat. He was negative for parasitic and bacterial infections, however presented low white blood cells and lymphopenia. The CT chest showed pneumonia with bilateral and subpleural areas of ground-glass opacification, consolidation affecting the lower lobes, thus confirming the diagnosis of COVID-19.
Although the relationship between urticaria and infection has rarely been reported, literature suggests that urticaria and angioedema can be induced by viral and bacterial infections. Urticaria has been associated before with Cytomegalovirus, Herpesvirus and Epstein-Barr virus. It was also found that once the viral infection was controlled, urticaria manifestations cleared up.
One study with 88 COVID-19 patients that analysed the cutaneous involvement found that 20,4 % presented with cutaneous manifestation, 8 of them developed it at disease onset, while 10 of the, developed it after hospitalisation. The cutaneous manifestations were erythematous rash, widespread urticaria and chickenpox-like vesicles.
Urticarial skin manifestations may be used as a possible diagnostic indicator in early COVID-19 stages.